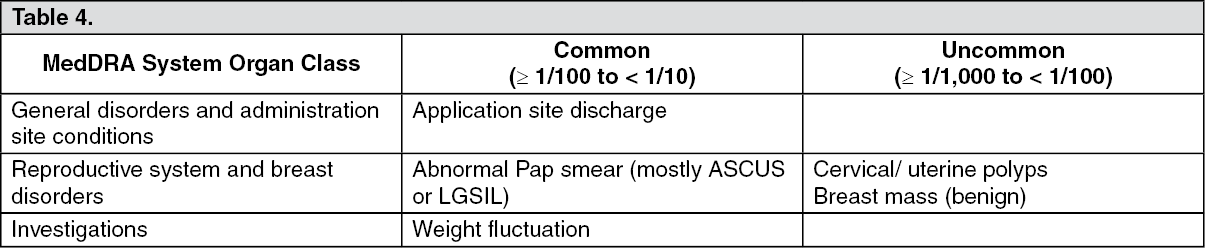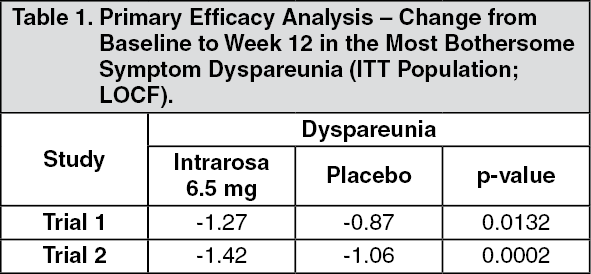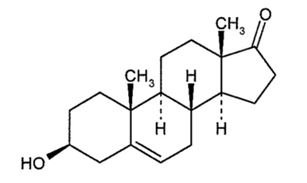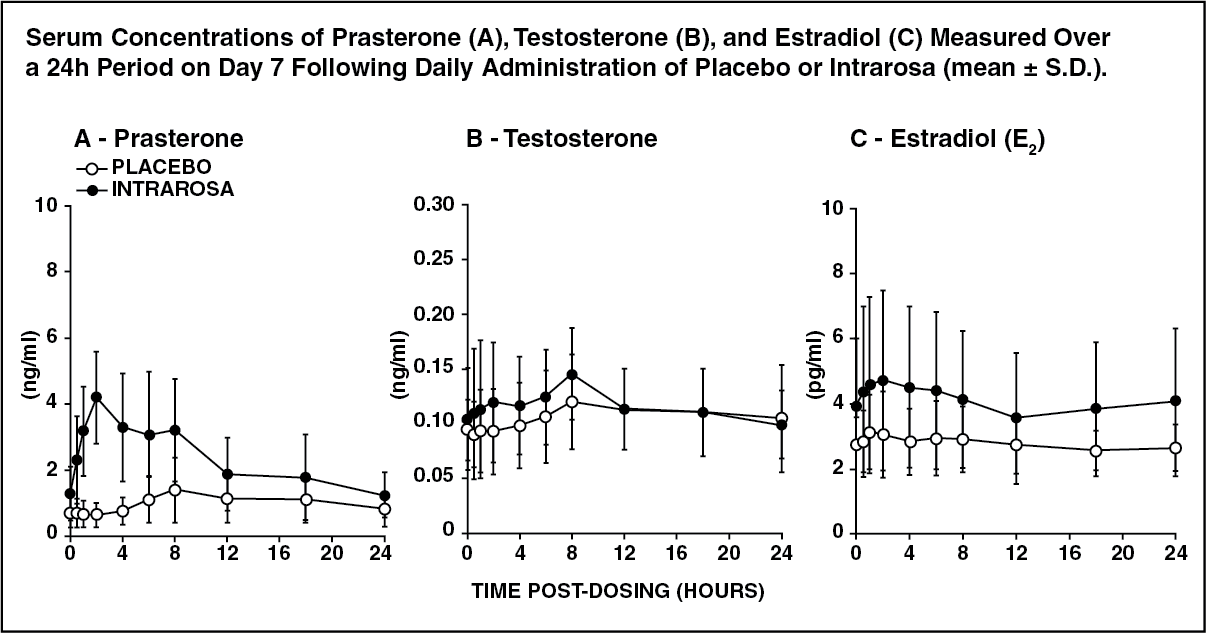
These highlights do not include all the information needed to use INTRAROSA® safely and effectively. See full prescribing information for INTRAROSA®. INTRAROSA® (prasterone) vaginal inserts Initial U.S. Approval: 2016

Lupin Pharma Canada announces the availability of Intrarosa® (prasterone vaginal ovules), a new treatment option in Canada for women with postmenopausal vulvovaginal atrophy